4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid
Name: 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid
CA Name: 2,7-Naphthalenedisulfonic acid,4-amino-5 -hydroxy- ,monsodium salt
Molecular Structure:
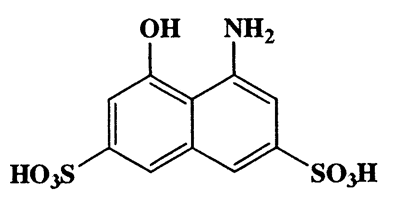
4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid,2,7-Naphthalenedisulfonic acid,4-amino-5 -hydroxy- ,monsodium salt,CAS 5460-09-3,319.31,C10H9NO7S2
Molecular Formula:C10H9NO7S2
Molecular Weight: 319.31
CAS Registry Number: 5460-09-3
List of synthetic dyes :

[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, 2-Aminobenzenesulfonic acid diazo, and […]
[…] Methods : aniline diazo, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and then and 2,4,6-Trichloro-1,3,5-triazine condensation […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidand 2,4,6-Trichloro-1,3,5-triazine condensation, 2-Aminobenzenesulfonic acid diazo, coupled with […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidand 2,4,6-Trichloro-1,3,5-triazine condensation, 2-Aminobenzenesulfonic acid diazo, coupled with […]
[…] Methods : Vomit’s acid diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcoupling, and 2,4,6-Trichloro-1,3,5-triazine condensation, again with 2-Amino-5-sulfobenzoic […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidand 2,4,6-Trichloro-1,3,5-triazine condensation, aniline diazo, and condensation products coupled, […]
[…] Methods : 2-Aminonaphthalene-1-sulfonic acid sulfonated, diazotization, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine first condensation, its product (2 Moore) and […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidand 2,4,6-Trichloro-1,3,5-triazine first condensation, and a second time between ester condensation, […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidand 2,4,6-Trichloro-1,3,5-triazine condensation chlorine, 2-Aminonaphthalene-1-sulfonic acid diazo, […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, 2-Aminonaphthalene-1-sulfonic acid diazo, and […]
[…] Methods : 2-Amino-5-methylbenzenesulfonic acid diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation, and then and N-ethylbenzenamine […]
[…] Methods : 2-(3-Amino-4-methoxyphenylsulfonyl)ethyl hydrogen sulfate diazo, and N acetyl 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and then the transformation of 1:1 copper […]
[…] Methods : 2-Amino-5-nitrophenol diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, reduction, and 5-Chloro-2,4,6-trifluoropyrimidine condensation and transformed into a […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine first condensation, 3-Amino-4-hydroxynaphthalene-1,5-disulfonic […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, and 5-Aminonaphthalen-1-ol in acetic acid medium […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, and 8-(P-toluidino)naphthalene-1-sulfonic […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, and 8-Aminonaphthalene-1-sulfonic acid […]
[…] Methods : 4-Nitrobenzenamine diazo, in acid conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, its product in alkaline conditions again with the diazo 4-(Phenyldiazenyl)benzenamine […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, in weak alkali conditions Coupled with resorcinol, 2-Amino-4,6-dinitrophenol diazo, in weak […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, Coupled with resorcinol, its products and 2 Moore’s diazo […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, Coupled with resorcinol, 4-Nitrobenzenamine diazo, and the generation of single […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, Coupled with resorcinol; 4-Nitrobenzenamine and 4-nitro-4′-aminoazobenzene-2-sulfonic […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic aciddiazo, Coupled with resorcinol, then respectively and diazo of 4-Nitrobenzenamine and […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic aciddiazo, Coupled with resorcinol, then respectively and diazo of 4-Nitrobenzenamine and […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo,and Resorcinol coupled, then respectively and diazo of 4-Nitrobenzenamine and […]
[…] Methods : 2-Methyl-5-nitrobenzenamine diazo, in acid conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcoupling, and then aniline diazo, in alkaline conditions and formation of single accidentally […]
[…] Methods : 4-(4-Aminophenyl)benzenamine double nitriding, in acid conditions coupled with 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, 2,5-Diaminobenzenesulfonic acid diazo, in alkaline conditions and the above the products of […]
[…] Methods : 4-(4-Aminophenyl)benzenamine double nitriding, in acid conditions coupled with 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, 2-Aminobenzenesulfonic acid diazo, in alkaline conditions and the product of the above-mentioned […]
[…] Methods : 4-(4-Aminophenyl)benzenamine double nitriding, first in acid conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and again between Benzene-1,4-diamine coupling,N-(4-aminophenyl)acetamide(2 Moore) […]
[…] Methods : 4-(4-Aminophenyl)benzenamine double nitriding, in acid conditions coupled with 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, 2-Aminobenzenesulfonic acid diazo, in alkaline conditions and the above the products of coupling, […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid diazo, and 2-Amino-4-methylanisole coupling, and then the light […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic aciddiazo, and 2,5-Dimethylbenzenamine coupling, its product to diazo, in alkaline conditions and […]
[…] Methods : 3,3′-Dimethoxylbenzidine double nitriding, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid(2 Moore) […]
[…] 4-(4-Aminophenyl)benzenamine double nitriding, first in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcoupling, again in alkaline conditions and accidentally coupled nitrogen […]
[…] Methods : 3,3′-Dimethylbenzidine double nitriding, in alkaline conditions first and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and then in alkaline conditions and 7-Amino-4-hydroxynaphthalene-2-sulfonic acid […]
[…] Methods : 4-(4-Aminophenyl)benzenamine double nitriding, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and then in alkaline conditions and 6-(Ethylamino)-4-hydroxynaphthalene-2-sulfonic […]
[…] Manufacturing Methods : aniline diazo, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling. […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, 4-Aminobenzoic acid diazo, and condensation […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, again with Nicotinic acid condensation, the […]
[…] Methods : 2-Aminonaphthalene-1,5-disulfonic acid diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation, and then and registration, […]
[…] : 5-Amino-2-(4-aminophenylamino)benzenesulfonic acid double nitriding, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid(2 Moore) coupling, and then and 2,4,6-Trichloro-1,3,5-triazine (2 Moore) condensation and […]
[…] acid diazo, Coupled with resorcinol, and its products are again diazo the 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 4-Nitrobenzenamine […]
[…] Methods : p-Methylaniline diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation, and […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine in turn and (1) 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, (2) 4-Nitrobenzenamine diazo, and 2-Hydroxybenzoic acid coupling, and then to restore the azo […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine successively with (1)4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, (2) 4-Nitrobenzenamine diazo, and 2-Hydroxybenzoic acid coupling, and then to restore the azo […]